Transport phenomena in the pervaporation process
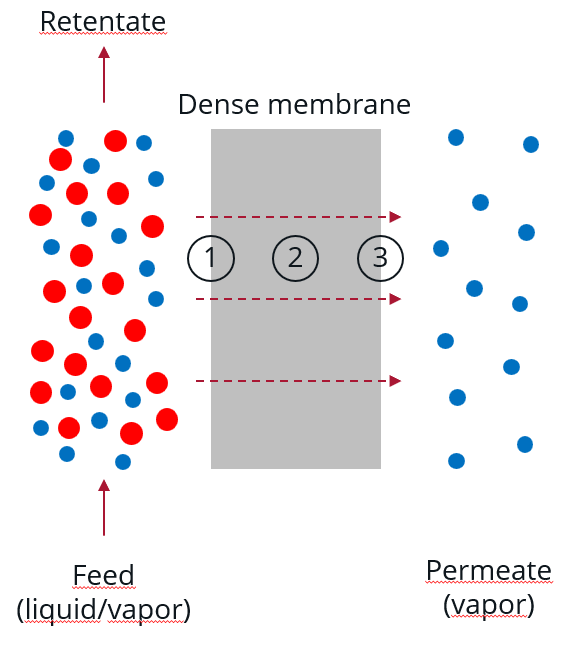
PERVAPORATION PRINCIPLES
General introduction on pervaporation

There are two main subject to be considered in selecting a dehydration technique: complexity of the process and energy demand.
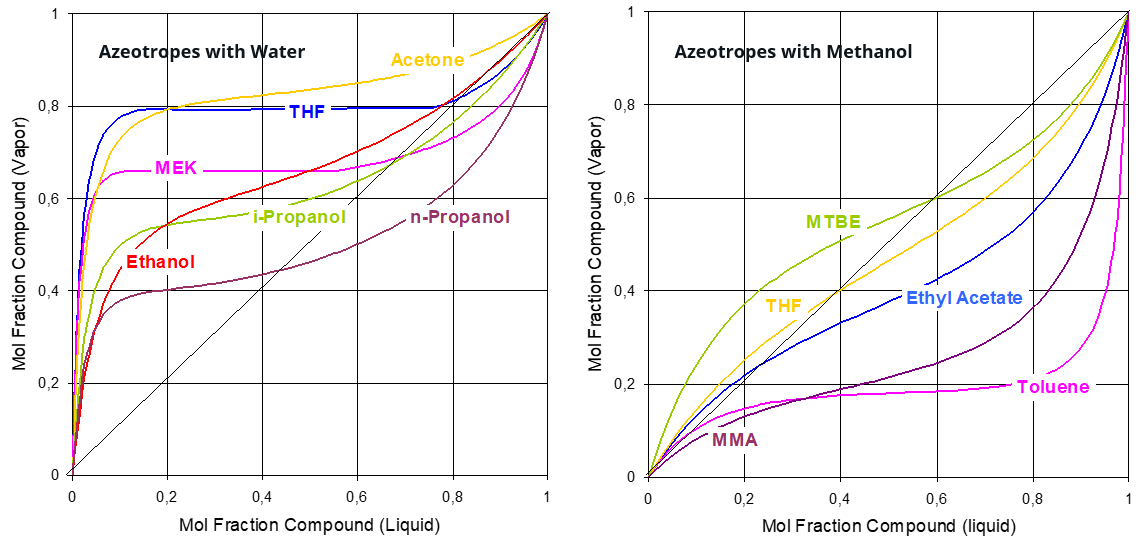
Most of the industrially relevant solvents form a non-ideal mixture with water. A relevant consequence of this nature is the existence of azeotropic compositions.
Examples of solvents which generate azeotropic mixtures with water include: ethanol, propanol, THF, butanol and many more. Such mixtures are hard to split with more traditional thermal separation processes like distillation, which get substantially more complex to achieve the separation. Complex distillation techniques used to split azeotropic mixutures include pressure swing distillation and ternary distillation (uses an additional component as entrainer). All the aformentioned complex thermal separation processes are energy intensive.
On the opposite side, the performance of a membrane process is totally unaffected by the existence of azeotropic compositions and can happen regardless of it. Furthermore, the energy consumption is substantially lower, being the amount of energy required corresponding only to the latent heat of the water to be removed.
Dehydration with membranes processes is simpler to implement and has a way lower energy demand in comparison to any alternative dehydration process.